Featured






From general-purpose LLMs to AI-native compliance workflows
Most compliance teams have already felt the jump: a general-purpose LLM (ChatGPT, Claude, Le Chat) can draft, translate, summarise, and structure information faster than any junior analyst. Used well, it is genuinely useful.

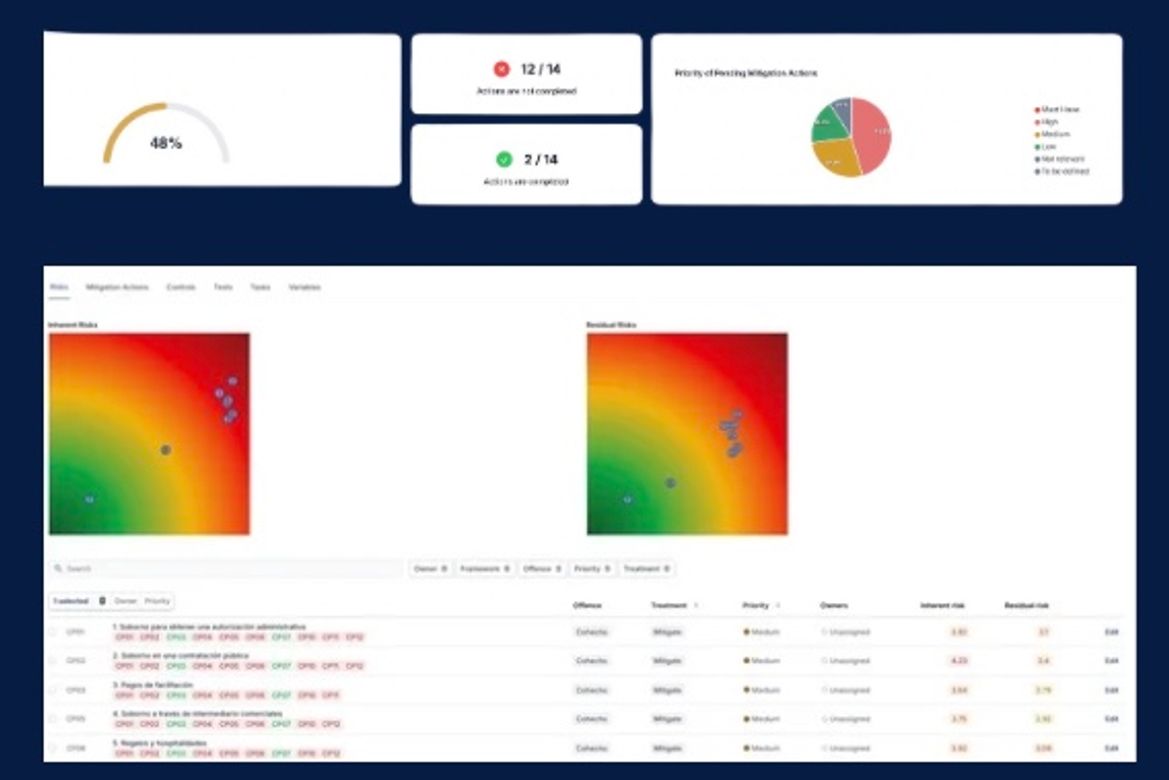
Policy management that auditors can test and trust
Most policy libraries fail in the same moment: when an auditor asks, “show me which version applied on that date, who approved it, who it applied to, who acknowledged it, and how you know it changed behavior.

Tone at the top: leadership in compliance risk management
Few elements of a compliance program change outcomes as decisively as tone at the top. Regulators and auditors consistently look for visible, sustained leadership that sets expectations, funds the work, and acts when it is inconvenient to do so.

DOJ FCPA priorities in 2025: what to expect in 2026
If you support a France or Spain headquartered group with any US touchpoints (US investors, US subsidiaries, USD payments, US-listed securities, or business routed through the US financial system), the US Department of Justice (DOJ) still matters for your anti-corruption program,

Managing the risks of gifts and hospitality, the AFA guide
Gifts and hospitality are one of the most common, and most misunderstood, sources of corruption risk. They sit at the intersection of commercial reality (maintaining relationships) and regulatory expectation (preventing undue influence).